Crystalline Structures
From DT Online
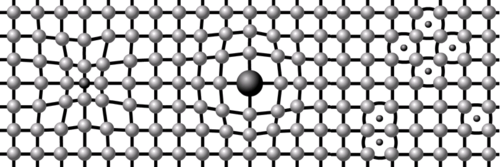
Different materials have different types and arrangements of Atoms and Molecules. The arrangement can be ordered and regular, as in the case of a Crystal, or more random to form a Glass or Ceramic.
As Crystalline Solids form, their atoms pack together as closely as possible - a little like bees creating a Honeycomb.
The ordered arrangements of atoms are called Crystal Lattices and they too can be based on hexagons - or cubes.
Metal atoms are packed together in one of three simple arrangements: Body-Centred Cubic; Face-Centred Cubic; or Close-Packed Hexagonal. These terms describe the repeating patterns, known as Unit Cells, which atoms organise themselves into

As with packing apples in a crate, if atoms are all similar size, an ordered and layered structure is easily obtained but, if of differing sizes, apples and oranges for example, the structure may remain ordered and regular but perhaps more distorted. It is these distortions in the structure that can change the physical properties of the resulting Alloy.
If the contributing atoms are much smaller, packing apples and grapes for example, it is possible they can fit into the spaces between the main metal atoms. This is the case of the alloy Steel in which Carbon atoms sit in between Iron atoms. Such arrangements are known as Interstitial Alloys.
Alloys are usually made by melting the main metal then stirring in a measured percentage of the additional element or elements until they dissolve - like adding salt to water. If the mixture solidifies without the additional elements separating out it is known as a Solid Solution and such Alloys (e.g. brass) are suitable for cold working.
Just as with adding salt to water however, there is a limit to how much of an additional element can be held in solution and, if this is exceeded, the excess separates out in the form of metalic compounds which lodge in the spaces between the main metal atoms - hence their name Intermetalic Compounds. These compounds are usually very Hard and Brittle and their presence results in an Alloy which generally has to be heated before it can worked. The heating and cooling has to be carried out carefully and over a limited temperature range.