Crystalline Structures
From DT Online
Different materials have different types and arrangements of Atoms and Molecules. The arrangement can be ordered and regular, as in the case of a Crystal, or more random to form a Glass or Ceramic.
As Crystalline Solids form, their atoms pack together as closely as possible - a little like bees creating a Honeycomb. The repeating patterns so formed are known as Unit Cells.
Cubic Lattices
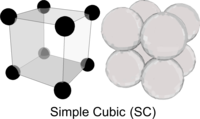
The ordered arrangements of atoms are called Crystal Lattices and they too can be based on hexagons - or cubes. The simplest way to arrange atoms in a cube lattice is to position one atom at each corner. This is known a Simple Cubic (SC) structure, as seen for common Salt. (Note: the SC lattice has eight atoms in total, each one contributing an eighth of its volume to the schematic cube as shown - therefore this particular Unit Cell holds just a single atom)
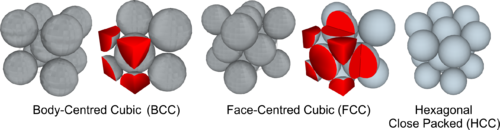
Metal atoms are packed together in one of three simple arrangements: Body-Centred Cubic; Face-Centred Cubic; or Hexagonal Close-Packed.
In addition to one atom at each corner, a Body Centred Cubic structure has an additional atom at the centre of the cube. The BCC lattice is the structure of Iron and Steel. (Note: as with the SC structure each corner atom contributes an eighth of its volume to the schematic cube, plus there is an atom at the centre, which means the BCC Unit Cell holds two atoms).
In addition to one atom at each corner, a Face Centred Cubic structure has an additional atom at the centre of each side or face of the cube. The FCC lattice is the structure which Iron and Steel change to when heated beyond a certain point. (Note: as with the SC structure each corner atom contributes an eighth of its volume to the schematic cube, plus there is a half atom contributed on each of the six sides, which means the FCC Unit Cell holds four atoms).
Structure of Alloys
Alloys are usually made by melting the main metal then stirring in a measured percentage of the additional element or elements until they dissolve - like adding salt to water. If the mixture solidifies without the additional elements separating out it is known as a Solid Solution and such Alloys are suitable for cold working.
As the mixture cools they may form into the crystal lattices in one of two common ways:
- If the alloying elements are nearly the same size (i.e. no more than 15% difference) then one element can be substituted by the other in the lattice structure - known as Solid Solution Alloys and are the most common. In such a circumstance, there is little or no distortion to the regular crystal lattice and the Alloys (e.g. Brass) are suitable for cold working.
- If the contributing atoms are much smaller, it is possible they can fit into the spaces between the main metal atoms. This is the case of the alloy Steel in which Carbon atoms sit in between Iron atoms. Such arrangements are known as Interstitial Alloys. Iron will absorb about 0.015% of Carbon at room temperature to form a Solid Solution known as Ferrite but all Steels contain more than this amount of Carbon.
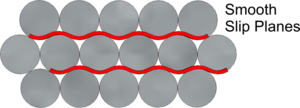
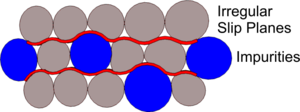
Pure metals do not usually have sufficient Strength for most purposes. As with packing apples in a crate, if atoms are all packed together in a neat structure, layers are formed between them and one layer can slide over another along what are known as Slip Planes - this is what makes metals easy to bend, Ductile and Malleable
If the atoms of different Alloying elements are of the same or similar size, an ordered and layered structure remains and the Slip Planes remain relatively smooth. But, if of differing sizes the structure may remain generally ordered but perhaps more distorted. It is these distortions in the structure, making the Slip Planes more irregular and less easy to slide over each other, that can change the physical properties of the resulting Alloy.
Dislocations
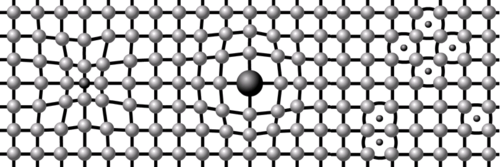
Crystal Lattices are distorted when an additional plane of atoms slides in between the regular structure. This allows the metal to be Plastically Deformed because planes of atoms can slide over each other. The line of additional atoms is known as a Dislocation and they slide through the Crystal Lattices in a wave motion until a Grain Boundary is reached. It follows that if the metal has a large Grains (e.g. they are are allowed to grow by slow cooling or Annealing) then the Dislocation can move more freely and the metal is Ductile and Malleable but if small Grains are generated (e.g. by rapid cooling or Quenching) the metal is Tougher and can be Brittle.
Work Hardening
It is a commonly experienced that if a piece of wire or thin metal sheet is repeated bent forwards and backwards it will eventually break. The bending or Working Hardening distorts the Crystal Lattice and is possible because the resulting Dislocations are able to move through it until they reach Grain Boundaries.
The Dislocations themselves however, can also inhibit the movement of further Dislocations by acting like additional Grain Boundaries. As the number of Dislocations increases by continued Cold Working, the metal therefore becomes Strengthened but eventually sufficiently Brittle to fracture. The lattice can be returned to its initial stress free state, with dislocations removed, by the process of Annealing.
Note: Working Hardening can be beneficial (e.g. cold forming bolt heads leaves them strong enough to be useful) but also potentially catastrophic. It is for this reason that Aluminium Alloy airframes are given a limited lifespan to ensure that the continuous vibration and flexing they are exposed to, does not embrittle them to the point of fracture.
Iron and Steel
Just as with adding salt to water however, there is a limit to how much of an additional element can be held in solution and, if this is exceeded, the excess separates out in the form of metallic compounds which lodge in the spaces between the main metal atoms - hence their name Intermetallic Compounds. These compounds are usually very Hard and Brittle and their presence results in an Alloy which generally has to be heated before it can be worked. The heating and cooling has to be carried out carefully and over a limited temperature range.
Pure Iron can be very soft and too weak for most purposes but its properties change significantly with the addition of small amounts of impurities. Iron is extracted from Iron Ore using a Blast Furnace. This Smelting process inevitably contaminates the Iron and the resulting Pig Iron can contain up to 4.5% Carbon in addition to other impurities. (Note: The output is known as Pig Iron because, originally, the iron was cast into sand moulds fed from a common runner. Because the groups of moulds resembled a litter of suckling pigs, the individual pieces of iron were referred to as Pigs and the runner was referred to as a Sow. Nowadays, Pigs are produced by a continuous casting machine).
The aim of Steelmaking processes such as the Bessemer Converter and Open Hearth Furnace (now superseded by Basic Oxygen Steelmaking and Electric Arc Furnaces) is to reduce these impurities and control the amount of Carbon which remains.
All Steels contain more than the very small amount of Carbon which will dissolve in Iron at room temperature. On cooling, excess Carbon separates out to form an Intermetallic Compound called Cementite or Iron Carbide. In Steel which has been Annealed (i.e. heated to above 9000C, its Critical Point, and allowed to cool slowly), the carbide compound can be viewed with a microscope as forming dark coloured areas layered against lighter coloured Ferrite areas. This mixture is known as Pearlite.
Cast iron has as much as 2% to 4% of Carbon. If all the excess remains as Cementite a very Brittle White Cast iron is formed but more usefully, between 1% and 3% of Silicon is also added to the melt, which causes the excess Carbon to form Graphite and results in Grey Cast iron. This has improved mechanical properties and the presence of Graphite makes the Iron self-lubricating and a good bearing metal which can be machined ‘dry’ without the use of a cutting fluid.
Tool Steel has a carbon content of between 0.5% and 1.5%. This amount of Carbon can only be held in solution with Iron at a high temperature when the Crystalline Structure changes from Body Centred Cubic to a Face Centred Cubic lattice and the dissolved Carbon forms Austenite - a material which only exists at these high temperatures. If the Steel is cooled or quenched suddenly, the excess Carbon is ‘frozen’ in solution to create a deformed BCC Crystalline Structure containing Martensite - a material too Hard and Brittle for most purposes.
Heat Treatment and Grain Structure
Tempering is a process which by controlled reheating enables the internal stress to be relieved and alters the size and distribution of carbides in the Martensite to improve Toughness at the expense of some Hardness.
Controlled Heat Treatment of metals in general determines their Crystalline Structure, or Grain Size, plus how and when the Alloying Elements or impurities precipitate. These changes can have quite dramatic effects upon the mechanical properties of some metals.