Density: Difference between revisions
From DT Online
Created Article |
mNo edit summary |
||
| Line 1: | Line 1: | ||
From everyday experience we know that some materials are lighter or heavier than others: that some materials float and others sink. The [https://en.wikipedia.org/wiki/Density '''Density'''] of a material describes this difference as the | From everyday experience we know that some materials are lighter or heavier than others: that some materials float and others sink. The [https://en.wikipedia.org/wiki/Density '''Density'''] of a material describes this difference as the [https://en.wikipedia.org/wiki/Mass '''Mass'''] per unit volume. It is calculated in [https://en.wikipedia.org/wiki/International_System_of_Units '''SI Units'''] by dividing a material's [https://en.wikipedia.org/wiki/Mass '''Mass'''] ''(in kilograms)'' by its volume ''(in cubic metres)'' but in practice is more usefully described as grams per cubic centimetre - which gives the same numerical value. | ||
[[File:DensityFormula.png|200px|center]] | [[File:DensityFormula.png|200px|center]] | ||
One cubic centimetre of water has a | One cubic centimetre of water has a [https://en.wikipedia.org/wiki/Mass '''Mass'''] of one gram and so the '''Density''' of water is one. Because of this, a cubic centimetre of water is the same as a millilitre so a millilitre of water also has a [https://en.wikipedia.org/wiki/Mass '''Mass'''] of one gram. Anything that floats in water will have a '''Density''' less than one, and more if it sinks. | ||
A further consequence is that the volume of an irregular object can be assessed by measuring the amount of water it displaces in millilitres when completely immersed and this is equal to its volume in cubic centimetres. The formulation of this relationship is famously attributed to [https://en.wikipedia.org/wiki/Archimedes '''Archimedes'''] when he noticed the water level rise as he got into his bath. | |||
The formulation of this relationship is famously attributed to [https://en.wikipedia.org/wiki/Archimedes '''Archimedes'''] when he noticed the water level rise as he got into his bath. | |||
[[Category:Terminology]] | [[Category:Terminology]] | ||
Latest revision as of 18:35, 30 December 2017
From everyday experience we know that some materials are lighter or heavier than others: that some materials float and others sink. The Density of a material describes this difference as the Mass per unit volume. It is calculated in SI Units by dividing a material's Mass (in kilograms) by its volume (in cubic metres) but in practice is more usefully described as grams per cubic centimetre - which gives the same numerical value.
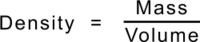
One cubic centimetre of water has a Mass of one gram and so the Density of water is one. Because of this, a cubic centimetre of water is the same as a millilitre so a millilitre of water also has a Mass of one gram. Anything that floats in water will have a Density less than one, and more if it sinks.
A further consequence is that the volume of an irregular object can be assessed by measuring the amount of water it displaces in millilitres when completely immersed and this is equal to its volume in cubic centimetres. The formulation of this relationship is famously attributed to Archimedes when he noticed the water level rise as he got into his bath.