Phase Diagrams
From DT Online
Phase Diagrams are charts which show how different Phases can exist together under certain conditions (e.g. the temperature range at which water and ice can exist together in slushy conditions). Three obvious Phases are the three states of matter: Solid, Liquid, and Gas but different Phases also exist when two or more liquids or solids come into contact with each other and form distinctive Crystalline Structures for example.
In Metallurgy we are normally concerned with just Solid and Liquid phases plus situations where these might exist together. The temperature line above which everything is Liquid is known as the Liquidus and the line below which everything is Solid is known as the Solidus.
Note: DT Online makes no attempt to explore the full depths of the fascinating topic of Metallurgy but hopes that the brief introduction given will stimulate interest and lead to further study. See the full series of lectures on YouTube (‘A’ Level Chemistry and beyond).
Cooling Curves
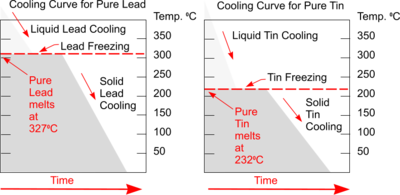
Plotting a graph of Temperature and Time as liquid metal cools will produce a Cooling Curve. Such graphs serve to illustrate the behaviour of metal as it cools and what changes occur at different temperatures.
Typical Cooling Curves for pure metals will be a stepped graph as shown. Heat is being lost throughout the process but at the point of freezing the temperature doesn't fall because the freezing process will liberate heat at exactly the same rate that it is being lost to the surroundings.
This is the result of energy being released when new atomic bonds are formed formed (e.g changing states from Liquid to Solid Lead or Tin).
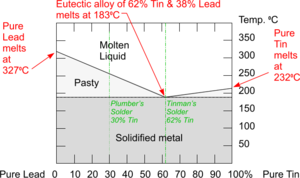
The Cooling Curve for an Alloy is more complicated and reveals interesting behaviours as solidification takes place.
With this particular Alloy, nothing happens at the normal freezing point of Lead because the addition of Tin has lowered this. Some solidification of Lead starts around 2500C but Tin remains liquid and there is not enough energy released to cause the Cooling Curve to go horizontal - although it does flatten out because cooling slows down a little.
The temperature stops falling at 1830C when both the Tin and Lead are starting to freeze. Once everything has solidified, the temperature continues to fall.
Phase Diagram for Lead/Tin Eutectic Alloy
Phase Diagrams combine the information from the Cooling Curves of mixtures of metals with metals and with other elements to create a chart which is used to show the behaviours of different Alloy compositions as they change phases between Solid to Liquid states.
The Phase Diagram for the Eutectic Alloy of 62% Tin & 38% Lead shows that at 1830C it changes from : Liquid to Solid without going through any ‘pasty’ stage which makes it ideal for Tinman's Solder.
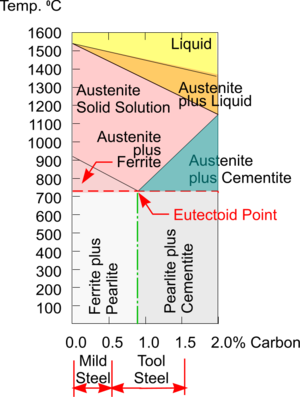
Phase Diagram for Carbon Steel
As Carbon Steels are heated towards their melting point their Crystalline Structures change from a Body Centred Cubic structure to a Face Centred Cubic structure. A consequence of this is that Iron heated above this Critical Point can absorb a lot more Carbon within a FCC structure.
Ferrite can be taken as just another name for Iron and, at room temperatures, it can absorb only a very small amount of Carbon (i.e. about 0.006%) within its single phase BCC structure.
But immediately above 723°C Iron changes to a different single phase FCC structure called Austenite and now can absorb up to about 0.8% carbon - and this increases with rising temperature. Austenite can only exist at this elevated temperature and so as it cools all the extra Carbon has to go somewhere.
Note: For simplicity the small area of single phase Ferrite on the left of the diagram has been omitted.
Ferrite, Cementite, Pearlite and Martensite
The Phase Diagram shows that for Steels with less than about 0.8% Carbon (i.e. Hypo Eutectic Steel) the mix solidifies into a two phase structure containing Ferrite which is very Soft and Ductile, and a layered structure of both Ferrite and Cementite (aka Iron Carbide) which is very Hard and Brittle - really a Ceramic. This layered structure is called Pearlite because of its appearance.
If cooled slowly, the Steel is stronger because of the added Cementite but still quite workable because large grains are given time to form and create space for Dislocations to move. The more Cementite the harder the metal, the more Ferrite, the softer it will be.
Steel with about 0.8% Carbon is known as Eutectoid Steel and at this concentration of Carbon, the grain structure is completely Pearlite. In Steel with more than about 0.8% of Carbon (i.e. Hyper Eutectoid Steel) there is too much Carbon to just form Pearlite and a mixture of Pearlite with additional Cementite precipitating out at the grain boundaries is formed.
If Austenite is Quenched there is no time for the Carbon to precipitate out and form Cementite, so the extra remains trapped in a deformed BCC structure called Martensite which is very Hard and Brittle such that, when a certain percentage of Carbon is exceeded, the Quenched steel can break like a stick of chalk. The more Carbon (e.g. up to about 2%) which is present, then the more Martensite is formed.
Note: Particular names are given to other compositions of Iron and Carbon which occur under certain time and temperature conditions e.g.:
- Ledeburite is a Eutectic alloy of Iron and Carbon containing about 4.3% Carbon.
- Bainite is a plate-like microstructure that forms in steels at temperatures of 250–550 °C
Tool Steel
When Austenite with a very low Carbon content is Quenched the resulting BCC structure can accommodate the extra Carbon atoms and no great distortion takes place so the Steel is relatively unaffected by this rapid cooling. It is for this reason that Mild Steel cannot be Hardened and Tempered but Tool Steel can.
Note:
The approximate, workshop ‘rule of thumb’, Carbon percentages for Mild Steel and Tool Steel are indicated but in reality, there are many much more precise SAE Grades of Carbon Steel available to suit the wide range of applications for this very versatile engineering material.
Cast Iron
Cast Iron is an Alloy of Iron and Carbon which contains more than 2% of Carbon.
Grey Cast Iron is the most commonly used Cast Iron. It is an Alloy of Iron and Carbon to which up to 3% Silicon is added. On cooling from its molten state, the additional Carbon is given time to combine with the Silicon and separate out to form flakes of Graphite within the Crystalline Structure. The Graphite flakes not only give rise to a grey appearance, hence the name, but are also self-lubricating which enables Grey Cast Iron to be machined ‘dry’ (i.e. without additional coolant).
By contrast, White Cast iron is cooled quickly, which suppresses the formation of Graphite and causes the extra Carbon to precipitate out as Cementite. As a consequence, White Cast iron is extremely Hard and too Brittle for most applications.
Malleable Cast Iron and Ductile Cast iron have a similar composition to White Cast iron with the exception that it is cooled more slowly via a controlled process which causes the surplus Carbon to form spheroidal particles of Graphite rather than flakes (i.e. Spheroidal Graphite). The rounded shape of the sphere-shaped Graphite inclusions at the grain boundaries reduce any Stress Concentration and limit the propagation of cracks.